The No Rinse Solution.
XPERIENCE® is a no rinse surgical solution, designed to help prevent surgical site infections by rinsing away debris and microorganisms.
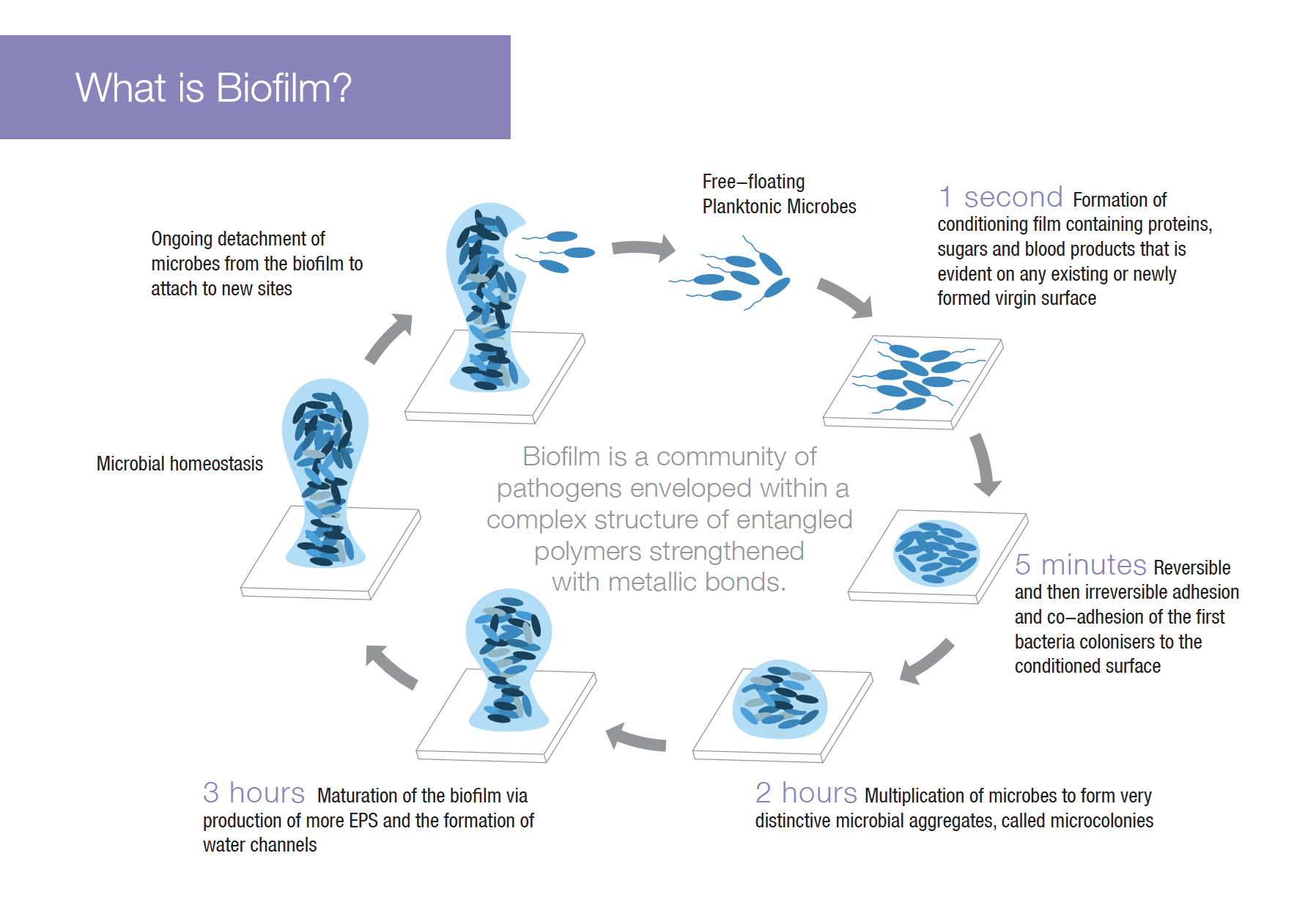
The technology deconstructs the biofilm that enters into the solution by removing the metal ions holding the polymers together and destroys the enveloped bacteria. XPERIENCE® defends against bacterial recolonisation.
Features
✔ 5+ hours of ongoing protection against bacterial biofilms*
✔ No secondary rinse out required
✔ Non-toxic
✔ Broad spectrum efficacy
✔ No known bacterial resistance
✔99.9% bacterial elimination
✔ Compatible with most commonly used implants and closure methods
XBIO® Technology
XBIO® Technology takes an innovative approach to solving the problem of bacterial biofilms.
Deconstruct the bacterial biofilm barrier
The unique, non-toxic technology attacks and deconstructs the structure of the biofilm by removing the metal ions that hold the EPS together.
Destroy the bacteria within, through cell lysis
The destruction of the biofilm barrier exposes the bacteria within the biofilm, making them more vulnerable to eradication. Bacteria that are enveloped within the XBIO® Technology are then destroyed by the combination of a surfactant and high osmotic imbalance across the bacterial cell wall.
Defend from recolonisation
XBIO® Technology’s broad spectrum efficacy helps defend from biofilm reformation, reducing the rate of reoccurrence by over 100 times. It is due to this unique mechanism of action that there is no known resistance to XBIO® Technology.
Collectively, infections contribute to significant morbidity, mortality and increased healthcare expenditures.
Proven efficacy against bacteria within the solution
Safety tested showing no irritation
XPERIENCE® has been safety tested showing no irritation.
The non-toxic formulation*
has been safety tested,
showing no irritation.
Proven Safety
Compatibility
-
WARNINGS/PRECAUTIONS:
NOT FOR IV USE - This product has been tested as a wound wash only. DO NOT inject.
External use only.
The product is single-use and should be applied only once in a 24-hour period.
Discard any unused solution.
Do not use if there is a history of allergy to any of the ingredients.
Avoid eye contact; product may cause ocular irritation.
Do not use if container is damaged.
Potential for temporary burning or discomfort may occur.
Do not use with fibrin sealants due to the risk of material degradation. Application of fibrin sealants in the presence of XPERIENCE® Advanced Surgical Irrigation may impact sealant setup.
If product contacts unintended anatomy or materials, rinse away solution after irrigation.
The Stryker StrykeFlow II laparoscopic irrigator does not provide adequate pressure to remove debris when used with XPERIENCE Advanced Surgical Irrigation.
Do not use in the case of substantial tissue loss.
Use of citrates may result in QT prolongation and other signs of short-term hypocalcaemia due to the ability for citrate to chelate ionised calcium.
Always read the label and follow the instructions for use.
This medical device must be administrated by a healthcare professional.
-
*Gupta K, Margques C, Petrova OE, Sauer K. Antimicrobial tolerance of Pseudomonas aeruginosa biofilms is activated during an early developmental stage and requires the two component hybrid sagS.
Journal of Bacteriology. 2013;195(21):4975-4987.
*Leid JG, Willson CJ, Shirtliff ME, et al. The exopolysaccharide
alginate protects Pseudomonas aeruginosa biofilm bacteria from
IFN-mediated macrophage killing. J Immunol. 2005;175(11):7512-7518.
*Williams, D. L. (2019, November 6). Targeting biofilms in translational research, device development, and industrial sectors. Google Books. Retrieved February 28, 2022, from
https://books.google.com/books?id=M8-8DwAAQBAJ
*Data on file: TR-04-21-008, TR-04-21-009
*Data on file: TR-06-19-012, TR-12-19-002
*Data on file: TR-06-19-007
*Data on file: TR-01-21-003, CD-0040